8 Organic Chemistry — Conformational Analysis: Cycloalkane Chair Drawing and Interpretation
Question
Consider: Trans 1,2-dibromocyclohexane
- Draw the simple line structure.
- Draw the two chair confirmations numbering the carbons and labelling the positions as axial/equatorial.
- Identify which is the more stable structure.
Show/Hide Answer
-
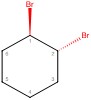
Simple line-dash-wedge structure - Chair conformations below:
-
- Conformation 1, bromine substituants in axial positions
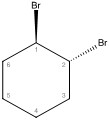
Chair conformation 1 with bromine substituants in axial positions - Conformation 2, bromine substituants in equatorial positions
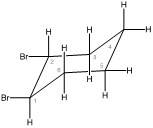
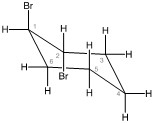
Chair conformation 2 with bromine substituants in equatorial positions
- Conformation 1, bromine substituants in axial positions
-
- The conformation with the bulkiest groups in equatorial positions is most stable; 1,3-diaxial interactions are minimized.
Refer to Section 9.4: Cyclohexane- A Strain-Free Cycloalkane (1).
Strategy Map
Do you need a little help to get started?
Check out the strategy map.
Show/Hide Strategy Map
| Strategy Map Steps |
|---|
| 1. Use the provided molecule name to draw its simple line structure. |
2. Draw the templates of the two possible conformations.
Show/Hide ResourceRefer to Section 9.4: Cyclohexane- A Strain-Free Cycloalkane (1). |
| 3. Number the carbons one through six. |
| 4. Label the axial and equatorial positions. |
| 5. Convert your simple line structure into your two templates. |
6. Compare the two conformations and identify which one would be the most stable.
Show/Hide HintThe most stable conformation is the one that has the least number of interactions between substituent atoms. The larger, more electronegative atoms are favoured in the equatorial positions as they will be impacted by less interactions with surrounding atoms. Refer to Section 9.5: Substituted Cyclohexanes (2). |
Solution
Do you want to see the steps to reach the answer?
Check out this solution.
Show/Hide Solution
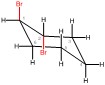
a. Simple Line Structure

This molecule is trans, meaning one bromine is dashed back, and one is wedged forward. These bromines are on a six-carbon ring and attached to carbons one and two.
b. Chair conformations
- Conformation 1 — Both bromines are in axial positions.

- Conformation 2 — Both bromines are in equatorial positions.

c. Conformation 2 is the most stable.
It has the least 1,3-diaxial interactions between the bromine groups and surrounding atoms.
Guided Solution
Do you want more help?
The guided solution below will give you the reasoning for each step to get your answer, with reminders and hints.
Show/Hide Guided Solution
| Guided Solution |
|---|
This question is a theory problem where you test your understanding of cycloalkane naming and chair conformations.
Show/Hide Resource
|
| Recall how to sketch a line structure:
1. Identify if the structure is Cis or Trans. Show/Hide Think About This!Trans 1,2-dibromocyclohexane 2. Determine if the parent chain is in the form of a chain or ring. (This will be at the end of the name). Show/Hide Don’t Forget!
|
How to draw the chair conformation:
Show/Hide Don’t Forget!
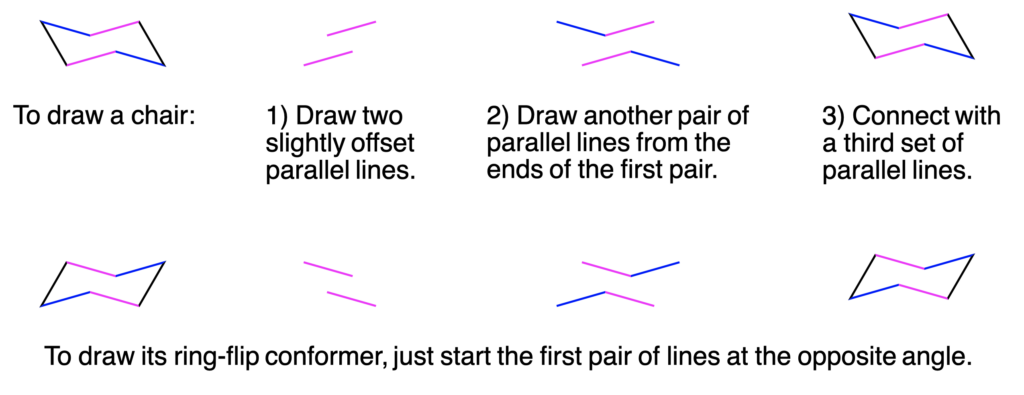 |
Identify the axial and equatorial positions. Identify if the structure is Cis or Trans.
Show/Hide Don’t Forget!Axial positions in red: 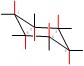
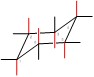
Equatorial Positions in blue: 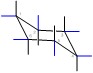
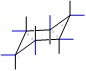
|
| Convert your simple line structure to your chair conformation:
1. Match the numbered carbons from your line structure to your template. 2. Recall that substituents wedged forward will be on the higher (‘up’) position and the substituent dashed backwards will be on the lower (‘down’) position. Show/Hide Watch Out!
 |
Identify the more stable conformation.
Show/Hide Don’t Forget!The most stable conformation is the one that has the least number of interactions between substituent atoms. The larger, more electronegative atoms are favoured in the equatorial positions as they will be impacted by fewer interactions with surrounding atoms. Show/Hide Resource
|
| Complete Solution |
|---|
| a. Simple Line Structure
This molecule is trans, meaning one bromine is dashed back and one is wedged forward. These bromines are on a six-carbon ring and attached to carbons one and two. |
| b. Chair Conformations
i. Conformation 1
Both bromines are in axial positions. The bromine on carbon one is in the highest position, and the bromine on carbon two is in the lowest position. ii. Conformation 2
Both bromines are in equatorial positions. All axial and equatorial positions switch. The bromine on carbon one is in the highest position, and the bromine on carbon two is in the lowest position. |
| c. The equatorial conformation is the most stable.
It has the fewest interactions between the bromine groups and surrounding atoms because they are both in equatorial positions. |
Check Your Work
To analyze if your answer is correct, evaluate which conformation has more equatorial positions and given the most space for any larger groups.
Why does this answer make chemical sense?
Show/Hide Answer
In the equatorial positions, the larger bromine atoms are the farthest away from all other substituent atoms. The more 1,3-diaxial interactions there are, the more energy the conformation requires, and the less stable the conformation would be.
PASS Attribution
- Question 9.E.2 from LibreText PASS Chemistry Book CHEM 1500 (3) is used under a CC BY-NC 4.0 license.
Media Attributions
- Simple Line Structure by Blackstock et al. from LibreTexts PASS Chemistry Book CHEM 1500 (3) is used under a CC BY-NC 4.0 license.
- Chair Conformation 1 by Blackstock et al. from LibreTexts PASS Chemistry Book CHEM 1500 (3) is used under a CC BY-NC 4.0 license.
- Chair Conformation 1 by Blackstock et al. from LibreTexts PASS Chemistry Book CHEM 1500 (3) is used under a CC BY-NC 4.0 license.
- Cyclohexane by Blackstock et al. from LibreTexts PASS Chemistry Book CHEM 1500 (3) is used under a CC BY-NC 4.0 license.
- Trans 1,2-dibromocyclohexane by Blackstock et al. from LibreTexts PASS Chemistry Book CHEM 1500 (3) is used under a CC BY-NC 4.0 license.
- How to Draw the Chair Conformation by Farmer et al. from LibreTexts Organic Chemistry (Morsch et al.) (4) is used under a CC BY-SA license.
- Axials Positions in Red 1 by Blackstock et al. from LibreTexts PASS Chemistry Book CHEM 1500 (3) is used under a CC BY-NC 4.0 license.
- Axials Positions in Red 2 by Blackstock et al. from LibreTexts PASS Chemistry Book CHEM 1500 (3) is used under a CC BY-NC 4.0 license.
- Equitorial Positions in Blue 1 by Blackstock et al. from LibreTexts PASS Chemistry Book CHEM 1500 (3) is used under a CC BY-NC 4.0 license.
- Equitorial Positions in Blue 2 by Blackstock et al. from LibreTexts PASS Chemistry Book CHEM 1500 (3) is used under a CC BY-NC 4.0 license.
- Simple Line Structure to Chair Conformation by Blackstock et al. from LibreTexts PASS Chemistry Book CHEM 1500 (3) is used under a CC BY-NC 4.0 license.
References
1. LibreTexts. 9.4: Cyclohexane- A Strain-Free Cycloalkane. In CHEM 1500: Chemical Bonding and Organic Chemistry. LibreTexts, 2023. https://chem.libretexts.org/Courses/Thompson_Rivers_University/CHEM1500%3A_Chemical_Bonding_and_Organic_Chemistry/09%3A_Organic_Chemistry_III_-_Conformational_Analysis/9.04%3A_Cyclohexane-_A_Strain-Free_Cycloalkane#How_to_Draw_the_Chair_Conformation.
2. LibreTexts. 9.5: Substituted Cyclohexanes. In CHEM 1500: Chemical Bonding and Organic Chemistry. LibreTexts, 2022. https://chem.libretexts.org/Courses/Thompson_Rivers_University/CHEM1500%3A_Chemical_Bonding_and_Organic_Chemistry/09%3A_Organic_Chemistry_III_-_Conformational_Analysis/9.05%3A_Substituted_Cyclohexanes.
3. Blackstock, L; Brewer, S.; Jensen, A. 9.2: Question 9.E.2 PASS – Cycloalkane Chair, Axial vs. Equatorial, Most Stable. In PASS Chemistry Book CHEM 1500. LibreTexts, 2023. https://chem.libretexts.org/Courses/Thompson_Rivers_University/PASS_Chemistry_Book_CHEM_1500/09%3A_Organic_Chemistry_III_-_Conformational_Analysis/9.03%3A_Question_9.E.2new_PASS_-_cycloalkane_chair_axial_vs._equatorial_most_stable.
4. Farmer, S.; Kennepohl, D.; Morsch, L.; Cunningham, K.; Reusch W., Bruner, R. 4.5: Conformations of Cyclohexane. In Organic Chemistry (Morsch et al.). LibreTexts, 2022. https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Organic_Chemistry_(Morsch_et_al.)/04%3A_Organic_Compounds-_Cycloalkanes_and_their_Stereochemistry/4.05%3A_Conformations_of_Cyclohexane.