10 Thermochemistry — Interpreting Thermochemical Equations and Sketching Enthalpy Diagrams
Question
Given the following thermochemical equation,
C6H6 (l) → 3C2H2 (g) ΔH = 630.0 kJ/mol.
Write the complete thermochemical equation for the reverse reaction. Sketch an enthalpy diagram showing the relative positions of all species and include arrows indicating both of the above reactions with appropriate values for ΔH reaction.
Show/Hide Answer
3C2H2 (g) → C6H6 (l)
ΔH = -630.0 kJ/mol
Refer to Section 3.4: Enthalpy of Reaction (1).
Strategy Map
Do you need a little help to get started?
Check out the strategy map.
Show/Hide Strategy Map
| Strategy Map Steps |
|---|
| 1. Identify the reactants and products for the forward reaction. |
2. Swap them to give the reverse reaction.
Show/Hide HintRecall that reversing a reaction changes the sign of ΔH. Refer to Section 3.4: Enthalpy of Reaction (1). |
3. Manipulate the enthalpy value from the forward reaction to give the value for the reverse reaction.
Show/Hide HintThe amount of energy will be the same regardless of the reaction’s direction. However, energy is absorbed in one direction and released in the other. |
| 4. Create the enthalpy diagram showing the reaction in both directions. |
Solution
Do you want to see the steps to reach the answer?
Check out this solution.
Show/Hide Solution
3C2H2 (g) → C6H6 (l)
The reactant and product from the forward reaction switch places.
ΔH = -630.0 kJ/mol
The sign of the enthalpy value for the forward reaction flips.
The thermochemical equation for the reverse reaction is:
3C2H2 (g) → C6H6 (l) ΔH = -630.0 kJ/mol
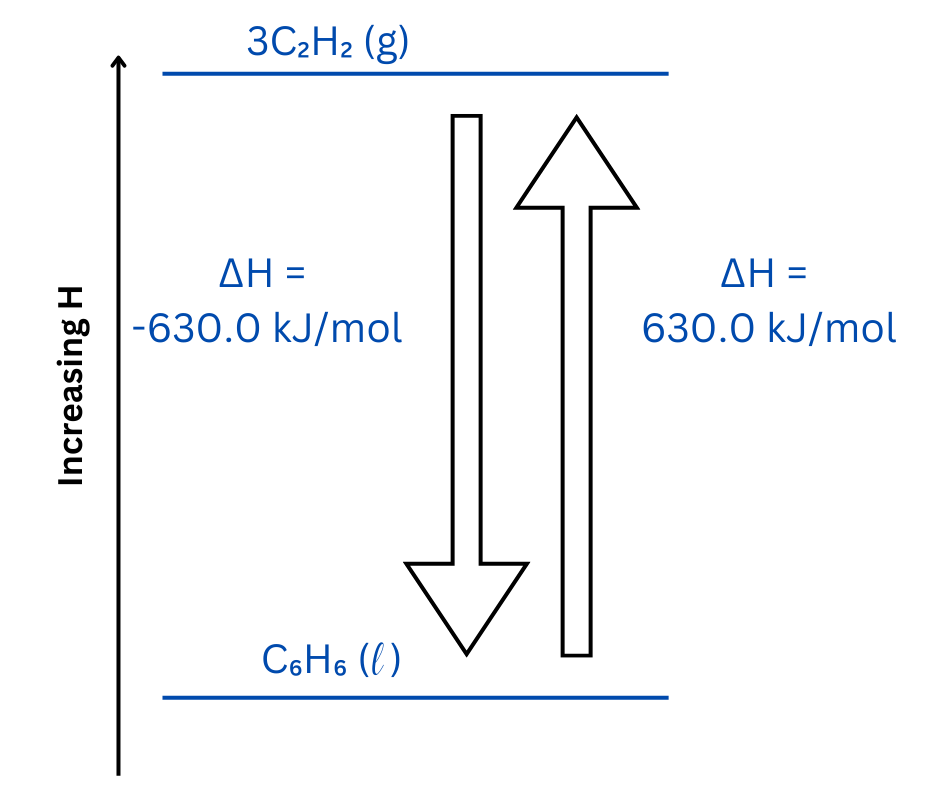
The enthalpy diagram shows the relative positions of all species and includes arrows indicating both of the above reactions with appropriate values for ΔH reaction.

The up arrow represents the forward reaction, and the down arrow represents the reverse reaction.
Refer to Section 3.4: Enthalpy of Reaction (1).
Guided Solution
Do you want more help?
The guided solution below will give you the reasoning for each step to get your answer, with reminders and hints.
Show/Hide Guided Solution
| Guided Solution Ideas |
|---|
This question is a theory problem that tests your knowledge of enthalpy. You must find the reverse reaction given its forward reaction, find its enthalpy and sketch the diagram representing the reaction in both directions.
Show/Hide ResourceRefer to Section 3.4: Enthalpy of Reaction (1). |
You have information about the forward reaction; how can it be manipulated to give the reverse reaction?
Show/Hide Think About This!Recall that if a reaction is reversed, the reactants and products switch sides, and the opposite reaction occurs. |
What about the enthalpy?
Show/Hide Don’t Forget!The magnitude of energy will be the same regardless of the reaction’s direction. However, energy is absorbed in one direction and released in the other. |
If the enthalpy change for the forward reaction is positive, what do you expect the enthalpy change for the reverse reaction to be?
Show/Hide Watch Out!If the enthalpy for the forward reaction is positive, you can expect the enthalpy for the reverse reaction to be negative. |
Recall how to sketch an enthalpy diagram:
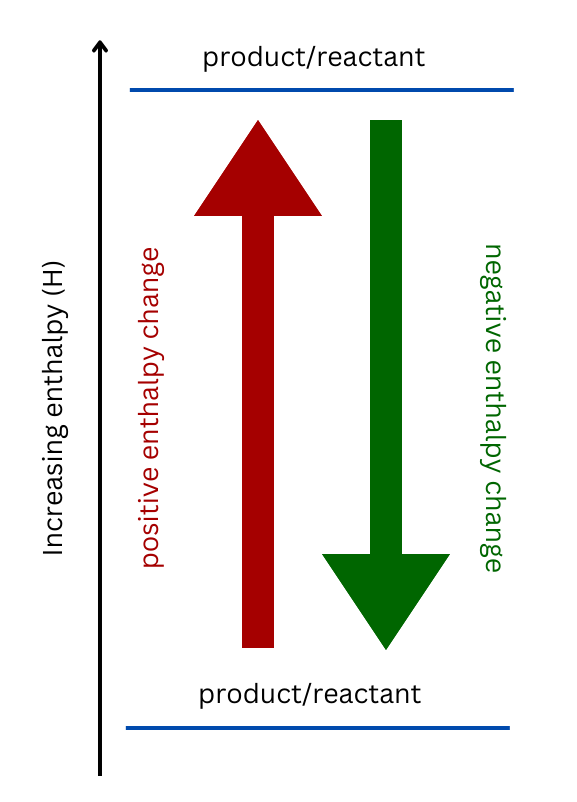
Show/Hide Watch Out!If the reaction is endothermic (meaning it has a positive enthalpy), the products will be higher than the reactants, and the reaction arrow will point upwards. The opposite is true for an exothermic reaction. Show/Hide Don’t Forget!
|
| Complete Solution |
|---|
| Given: C6H6 (l) → 3C2H2 (g) ΔH = 630.0 kJ/mol
Reverse the reaction The reactant and product from the forward reaction switch places. In the forward reaction three C2H2 molecules break to form one C6H6 molecule. In the reverse reaction one C6H6 molecule is broken to form three C2H2 molecules. |
| Change the sign of ΔH ΔH = 630.0 kJ/molThe sign of the enthalpy value for the forward reaction is flipped to give the enthalpy value of the reverse reaction. This will always be the case since one way is endothermic while the other is exothermic. |
| Now, use the enthalpy diagram template to position the reactant and product, paying attention to the sign of ΔH in each case to determine relative positions.
The forward and reverse reactions are placed on the enthalpy diagram. The upward arrow represents the forward reaction, and the downward arrow represents the reverse reaction. The forward reaction is endothermic meaning it requires an input of energy. The reverse reaction is an exothermic reaction meaning energy is released from the reaction. |
| Answer: The complete thermochemical equation for the reverse reaction is therefore:
3C2H2 (g) → C6H6 (l) ΔH = -630.0 kJ/mol |
| Both reactions are represented on the enthalpy diagram:
|
Check Your Work
A complete thermochemical equation has a reactant, product and value of ΔH.
This question involves an endothermic reaction, represented by the up arrow on the enthalpy diagram. The reverse reaction is exothermic and represented as the up arrow.
Watch Out!
Make sure your ‘down’ arrow represents the exothermic reaction and your ‘up’ arrow represents the endothermic reaction.
Does your answer make chemical sense?
Show/Hide Answer
For a chemical reaction, the enthalpy of the reaction (ΔHrxn) is the difference in enthalpy between products and reactants. Reversing a chemical reaction reverses the sign of the enthalpy. This reversal makes sense because a reaction occurring in one direction will be either exothermic or endothermic when the bonds break and form.
Therefore, if the same reaction happens but the opposite bonds break and form, the enthalpy will be in the opposite direction. Because of this, if the forward reaction is endothermic, the reverse reaction will be exothermic and vice versa.
PASS Attribution
- LibreTexts PASS Chemistry Book CHEM 1510/1520 (2).
- Question 3.E.10 from LibreTexts TRU: Fundamentals and Principles of Chemistry (CHEM 1510 and CHEM 1520) (3) is used and modified under a CC BY-NC-SA 3.0 license.
Media Attributions
- Figures 1 & 2, by the authors (Brewer S. and Blackstock L.) are free to use under a CC0 license.
References
1. Thompson Rivers University. 3.4: Enthalpy of Reaction. In TRU: Fundamentals and Principles of Chemistry (CHEM 1510 and CHEM 1520). LibreTexts, 2022. https://chem.libretexts.org/Courses/Thompson_Rivers_University/TRU%3A_Fundamentals_and_Principles_of_Chemistry_(CHEM_1510_and_CHEM_1520)/03%3A_Thermochemistry/3.04%3A_Enthalpy_of_Reaction.
2. Blackstock, L.; Brewer, S.; Jensen, A. In PASS Chemistry Book CHEM 1510/1520; LibreTexts, 2023. https://chem.libretexts.org/Courses/Thompson_Rivers_University/PASS_Chemistry_Book_CHEM_1510%2F%2F1520.
3. Thompson Rivers University. 3.E: Thermochemistry (Exercises). In TRU: Fundamentals and Principles of Chemistry (CHEM 1510 and CHEM 1520). LibreTexts, 2023. https://chem.libretexts.org/Courses/Thompson_Rivers_University/TRU%3A_Fundamentals_and_Principles_of_Chemistry_(CHEM_1510_and_CHEM_1520)/03%3A_Thermochemistry/3.E%3A_Thermochemistry_(Exercises).