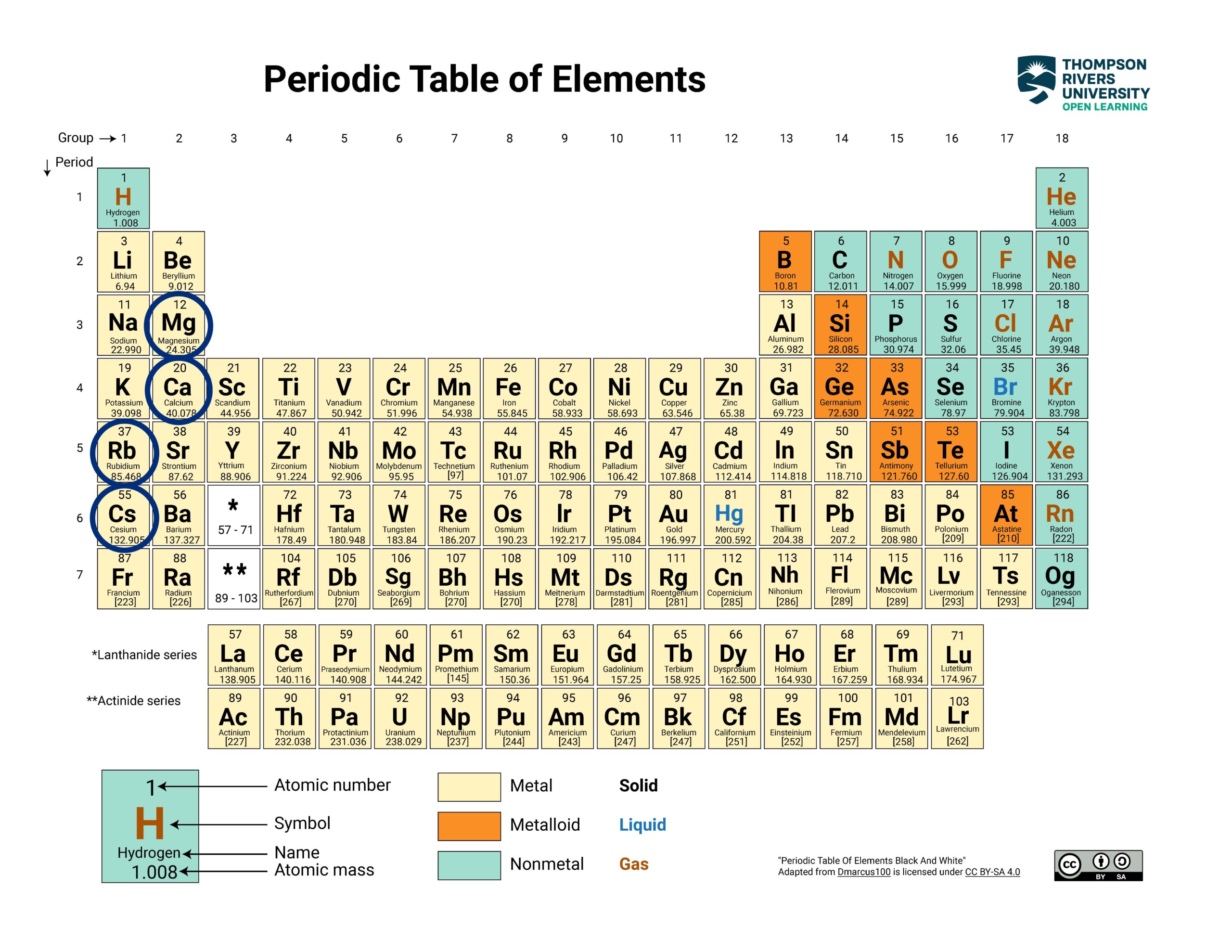
2 Periodic Relationships Among the Elements — Ranking Atomic Radius Size
Question
Based on their positions in the periodic table, list the following atoms in order of increasing radius: Ca, Cs, Mg, Rb.
Show/Hide Answer
Mg, Ca, Rb, Cs
Refer to Section 3.2: Periodic Variations in Element Properties (1).
Strategy Map
Do you need a little help to get started?
Check out the strategy map.
Show/Hide Strategy Map
| Strategy Map Steps |
|---|
1. Recognize the context of the question and confirm the requested format of the answer.
Show/Hide HintThis question requires an understanding of atomic radii. You must list these elements in order from smallest to largest atomic radii. Refer to Section 3.2.2: Variation in Covalent Radius (1). |
2. Find each element on the periodic table.
Show/Hide Periodic Table
|
3. Recall relevant concepts that impact atomic radii. Summarize the periodic trends to sort them into descending or ascending sizes.
Show/Hide HintConsider effective nuclear charge (Zeff). Refer to Section 3.2.1 Effective Nuclear Charge: Penetration and Shielding (1). Atomic radii increase as you move down a period and decrease as you move right across a group.
|
Solution
Do you want to see the steps to reach the answer?
Check out this solution.
Show/Hide Solution
The column (group) and period (row) locations of Ca, Cs, Mg, and Rb on the periodic table:
- Ca — atomic number 20, group 2, period 4
- Cs — atomic number 55, group 1, period 6
- Mg — atomic number 12, group 2, period 3
- Rb — atomic number 37, group 1, period 5
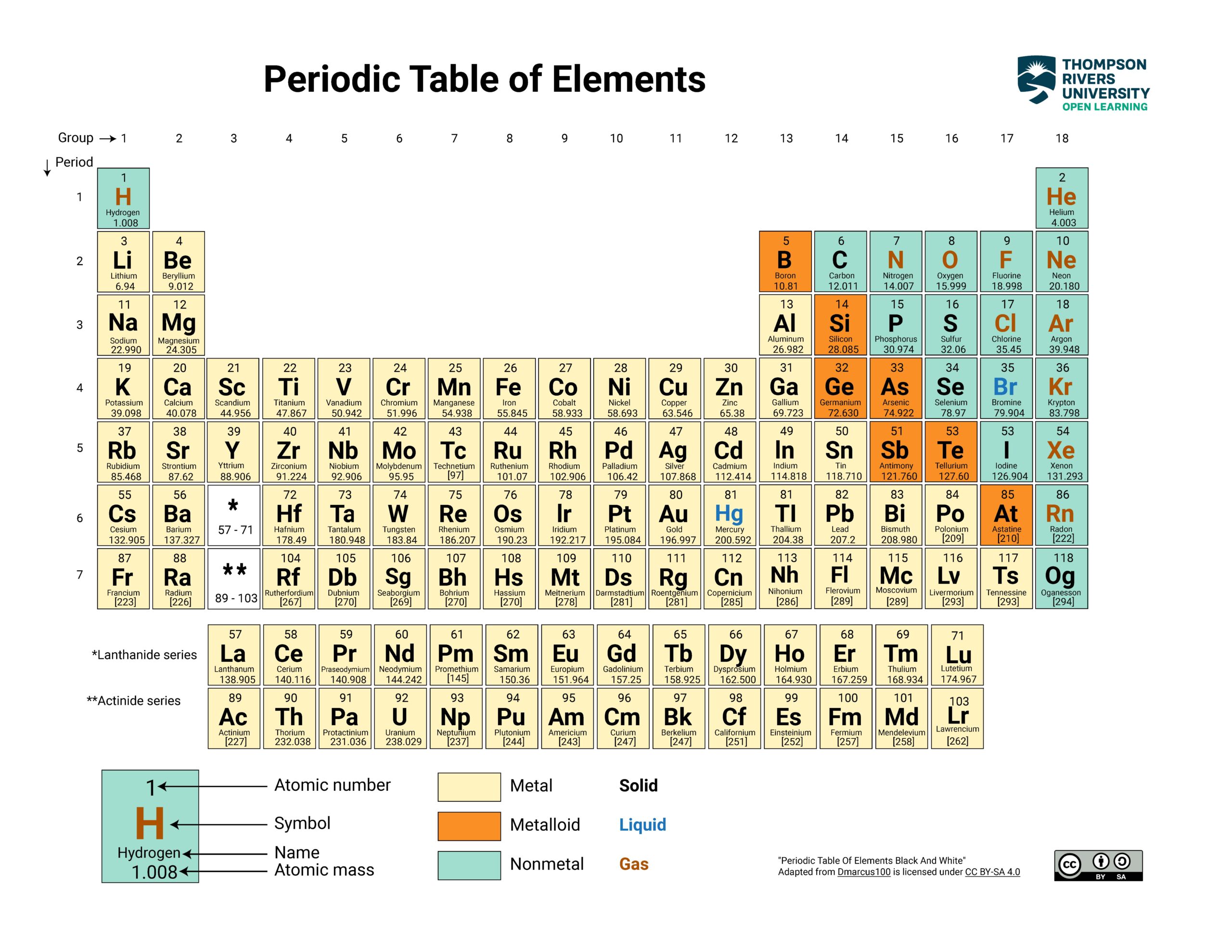
Smallest to largest atomic radii: Mg < Ca < Rb < Cs
Answer (in order of increasing radius): Mg, Ca, Rb, Cs
Guided Solution
Do you want more help?
The guided solution below will give you the reasoning for each step to get your answer, with reminders and hints.
Show/Hide Guided Solution
| Guided Solution Ideas |
|---|
| This question is a theory problem that requires the understanding and application of concepts related to the shape of atomic orbitals, effective nuclear charge, and atomic radii.
This question asks us to rank the given elements by their size. Refer to Atomic Radii (2). Show/Hide Don’t Forget!Recognize that as principal quantum number (n) increases (i.e., as you move down the periodic table), the size of the orbital increases. Refer to Section 2.5: The Shape of Atomic Orbitals (3). Show/Hide Don’t Forget!Recognize that as Zeff increases (i.e., as you move left to right across the periodic table), the size of the orbital decreases. Refer to: |
Consider what characteristics may influence the distance between the nucleus and valence electron(s).
Show/Hide Think About This!Principal quantum number (n):
Effective Nuclear Charge (Zeff):
Show/Hide Don’t Forget!(n) influences size more than (Zeff). |
Summarize the trend of atomic radii across the periodic table.
Show/Hide Don’t Forget!The atomic radius decreases from left to right and increases from top to bottom.
|
Locate the elements on the periodic table
Show/Hide Element Locations
|
List the elements in order of increasing principal quantum number (n).
Show/Hide Don’t Forget!As (n) increases, the size of the orbital increases. Recall: The valence electrons for all of the listed elements are in s-orbitals:
Note that because all the elements are in different periods, we do not need to consider their group location (i.e., relative position from left to right). Refer to Section 3.2.2: Variation in Covalent Radius (1). |
| Complete Solution |
|---|
| Atomic radius increases as you move down rows from the top to the bottom of the periodic table.
This increase is because the principal quantum number (n) increases, meaning the element requires more shells to house additional electrons around the nucleus. Elements at the bottom of the periodic table have a larger ‘period’ number. |
| Atomic radius decreases as you move from left to right across the columns of the periodic table.
This decrease is because the effective nuclear charge (Zeff) increases, meaning that outer shell valence electrons feel a stronger attractive force from the protons that pull them closer to the nucleus. Elements towards the right of the periodic table have a larger ‘group’ number. |
Because of the left-to-right trend, the atomic number (i.e., the number of electrons) is not the only factor to consider when ranking atomic size.
|
Locate each element on the periodic table to find their relative period and group numbers.
|
Because each listed element is in a different row (i.e., period), they can be ranked for size using this information alone.
Smallest to largest atomic radii: Mg, Ca, Rb, Cs |
Check Your Work
The element at the bottom left (Fr: Francium) in the periodic table is the largest, and the element at the top right (He: Helium) is the smallest.
From the provided list, Mg is closest to the top and should be listed first; Cs is nearest to the bottom and should be listed last. No two elements are in the same period (row), so you do not need to consider the group (column) number in this question.
Does your answer make chemical sense?
Show/Hide Answer
In general, atomic radii increase as you move from top to bottom on the periodic table. It makes sense that size increases as the principal quantum number (n) increases. The periodic table is arranged logically, with each row representing an increased (n). As the ‘n’ value increases, the size of the orbital increases. This is because there are complete shells (i.e., core electrons) between the nucleus and the valence electrons. The core electrons effectively shield the valence electrons from the positive force. Therefore, the more core electrons there are, the weaker the attractive force; the valence electrons are held more weakly (i.e., at a further distance) from the nucleus, resulting in increased atomic radii.
PASS Attribution
- LibreTexts PASS Chemistry Book CHEM 1510/1520 (4)
- Question 3.E.9 from LibreTexts PASS Chemistry Book CHEM 1500 (5) is used under a CC BY-SA 4.0 license.
- Question 3.E.9 is question Q6.5.9 from LibreTexts Chemistry 1e (OpenSTAX) (6), which is under a CC BY 4.0 license.
- Question Q6.5.9 is question 75 from OpenStax Chemistry 2e (7), which is under a CC BY 4.0 license. Access for free at https://openstax.org/books/chemistry-2e/pages/1-introduction.
Media Attributions
- Figure 1: Periodic Table of Elements Black and White [adapted from Dmarcus100] by Thompson Rivers University Open Learning (8) is used under a CC BY-SA 4.0 license.
- Figure 2: [Modified] Periodic Table Of Elements Black and White [adapted from Dmarcus100] by Thompson Rivers University Open Learning (8) is used and modified under a CC BY-SA 4.0 license.
References
1. OpenStax. 3.2: Periodic Variations in Element Properties. In CHEM 1500: Chemical Bonding and Organic Chemistry. LibreTexts, 2023. https://chem.libretexts.org/Courses/Thompson_Rivers_University/CHEM1500%3A_Chemical_Bonding_and_Organic_Chemistry/03%3A_Periodic_Relationships_Among_the_Elements/3.02%3A_Periodic_Variations_in_Element_Properties#Effective_Nuclear_Charge:_Penetration_and_Shielding.
2. LibreTexts. Atomic Radii. In Supplemental Modules (Physical and Theoretical Chemistry). LibreTexts, 2023. https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Physical_Properties_of_Matter/Atomic_and_Molecular_Properties/Atomic_Radii.
3. Blackstock, L.; Brewer, S.; Cinel, B.; Prema, D. 2.5: The Shape of Atomic Orbitals. In CHEM1500: Chemical Bonding and Organic Chemistry. LibreTexts, 2022. https://chem.libretexts.org/Courses/Thompson_Rivers_University/CHEM1500%3A_Chemical_Bonding_and_Organic_Chemistry/02%3A_Quantum_Theory_and_Electronic_Structure_of_Atoms/2.05%3A_The_Shape_of_Atomic_Orbitals.
4. Blackstock, L.; Brewer, S.; Jensen, A. PASS Chemistry Book CHEM 1510/1520; LibreTexts, 2023. https://chem.libretexts.org/Courses/Thompson_Rivers_University/PASS_Chemistry_Book_CHEM_1510%2F%2F1520.
5. Blackstock, L.; Brewer, S.; Jensen, A. 3.2: Question 3.E.09 PASS – Ranking Atomic Radius. In PASS Chemistry Book CHEM 1500. LibreTexts, 2024. https://chem.libretexts.org/Courses/Thompson_Rivers_University/PASS_Chemistry_Book_CHEM_1500/03%3A_Periodic_Relationships_Among_the_Elements/3.02%3A_Question_3.E.09_PASS_-_ranking_atomic_radius.
6. OpenStax. 6.E: Electronic Structure and Periodic Properties (Exercises). In Chemistry 1e (OpenSTAX). LibreTexts, 2022. https://chem.libretexts.org/Bookshelves/General_Chemistry/Chemistry_1e_(OpenSTAX)/06%3A_Electronic_Structure_and_Periodic_Properties_of_Elements/6.E%3A_Electronic_Structure_and_Periodic_Properties_(Exercises).
7. Flowers, P.; Theopold, K; Langley, R.; Robinson, W. R. Ch. 6 Exercises. In Chemistry 2e. OpenStax, 2019. https://openstax.org/books/chemistry-2e/pages/6-exercises.
8. Thompson Rivers University Open Learning. Periodic Table Of Elements Black And White [adapted from Dmarcus100].